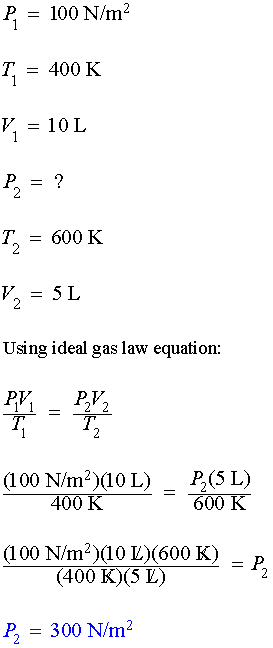Word Problems: Ideal Gas Law
For ideal gas law word problems, it is beneficial to present the general ideal gas law equation:

where:

- P1 = Initial Pressure
- V1 = Initial Volume
- T1 = Initial Temperature
- P2 = Final Pressure
- V2 = Final Volume
- T2 = Final Temperature
Example #1:
Solution #1:
An
ideal gas possesses an initial pressure of 200 Newtons per square meter
at a temperature of 1000 Kelvin. While holding the volume
constant, the temperature is decreased to 400 Kelvin. What is the
resultant pressure?
Solution #1:

Example #2:
Ten
liters of an
ideal gas possess an initial pressure of 100 Newtons per square meter
at a temperature of 400 Kelvin. If the volume is decreased to
five liters while raising the temperature to 600 Kelvin, what is the
resultant pressure?
Solution #2: